
Meaning Of Ph Level. Ph is the negative log of hydrogen ion concentration in a water based solution. Acidic solutions solutions with higher concentrations of h ions are measured to have lower ph values than basic or alkaline solutions. On this scale the strongest acid is 0 and the strongest alkali is 14. An expression widely used in medicine of the acidity or alkalinity of a solution.
The term ph was first described by danish biochemist søren peter lauritz sørensen in 1909. The universal indicator turns a different colour for all the numbers on the ph scale. The ph scale measures how acidic or alkaline basic something is. It s a lot easier to use a logarithmic scale instead of always having to write down all those zeros. P otential of h ydrogen american heritage dictionary of the english language fifth edition. Acidic solutions solutions with higher concentrations of h ions are measured to have lower ph values than basic or alkaline solutions.
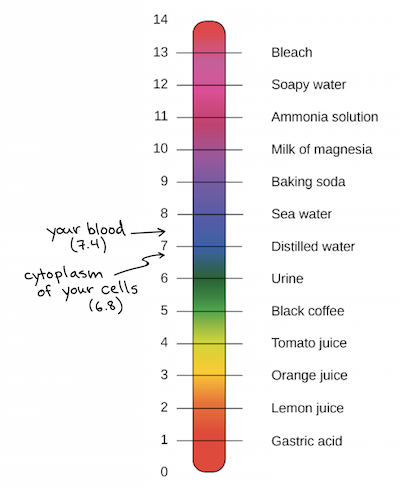
Your body works constantly to carefully control ph levels of blood and other fluids.
The ph scale commonly in use ranges from 0 to 14. Your body works constantly to carefully control ph levels of blood and other fluids. Aqueous solutions at 25 c with a ph less than 7 are acidic while those with a ph greater than 7 are basic or alkaline. Acidic solutions solutions with higher concentrations of h ions are measured to have lower ph values than basic or alkaline solutions. Ph quantitative measure of the acidity or basicity of aqueous or other liquid solutions. The ph scale usually ranges from 0 to 14.