
Acid Based Balance. Acid base balance normal ph. A buffer solution is a mixture of a weak acid and its conjugate base or a weak base and its conjugate acid. Acid base balance refers to the balance between input intake and production and output elimination of hydrogen ion. Disorders of acid base balance can lead to severe complications in many disease states.
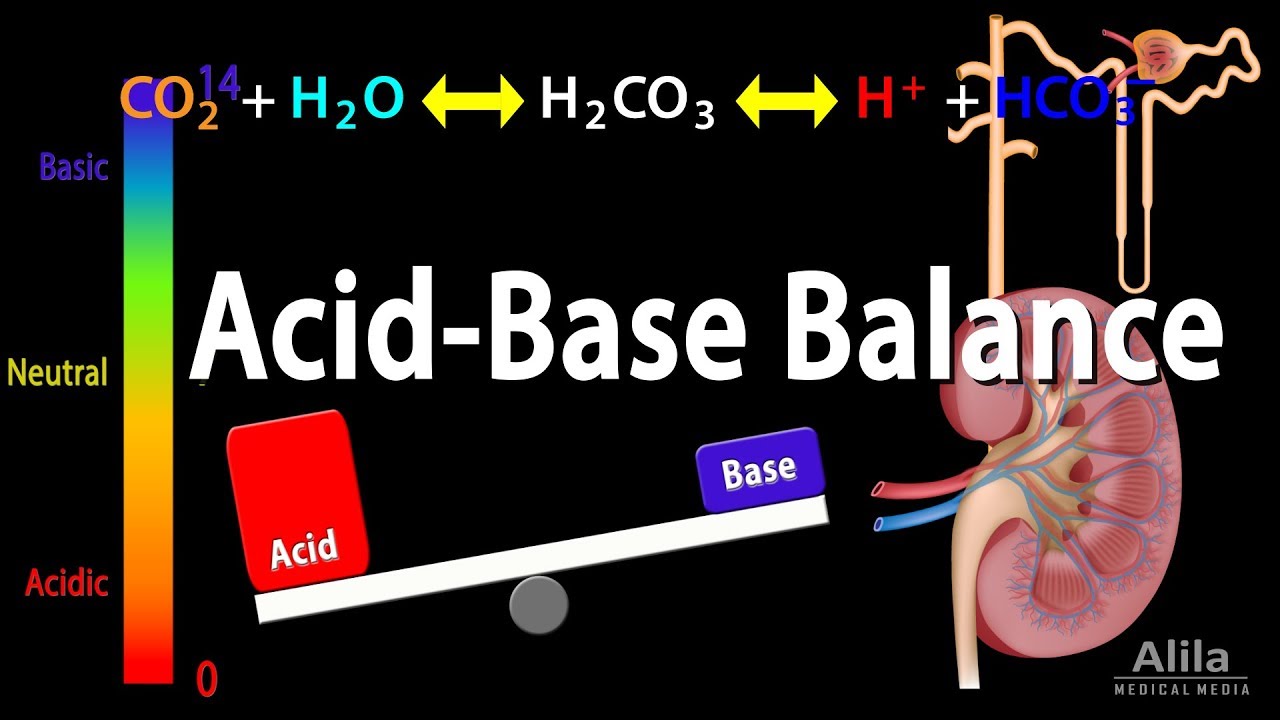
Buffer solutions keep the ph constant in a wide variety of chemical actions. 7 35 7 45 acidosis physiological state resulting from abnormally low plasma ph alkalosis physiological state resulting from abnormally high plasma ph acidemia. Acidic too much acid lower than 7 35 means that there is too much acid in your system. Maintaining the ph within these limits is achieved by bicarbonate other buffers the lungs and the kidneys. Hydrogen containing substances which dissociate in solution to release h any ionic or molecular substance that can act as a proton h donor. Plasma ph 7 45.
Strong acid hcl h2so4 h3po4.
Alkaline not enough acid it just means not acidic enough. Strong acid hcl h2so4 h3po4. Plasma ph 7 35 alkalemia. One of these is maintaining an acid base balance. To maintain homeostasis the human body employs many physiological adaptations. Your blood needs the right balance of acidic and basic alkaline compounds to function properly.